Written by Dentistry TodayFriday, 24 May 2013 08:09
 |
The Food and Drug Administration may soon lower the risk class of blade-form dental implants.
An advisory panel will meet in July to analyze the case of lowering the blade-form endosseous dental implants from the highest risk category to a lower one.
The FDA will look at reclassifying Class III devices into the Class II category. The FDA believes that certain controls can effectively monitor the risks associated with these types of implants. These devices have been discussed for numerous years.
The devices have displayed a high success rate in regards to staying implanted in the mouth without the necessity for removal. It also appears as though these implants have the ability to hold up over time.
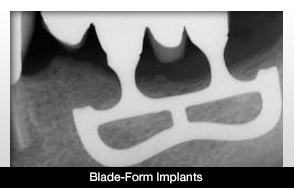
The dental blade-form implant was created to be put in place of tooth roots, in addition to providing support in the gingival tissue through the oral cavity. The goal is to allow the possibility for chewing. These implants are rectangular-shaped with a narrow edge. The implants are often made of titanium.
These implants are one of the few Class III devices left. They were first placed into that category because they are implanted in the body and can cause pain at any moment based on nerve impingement or bone issues in the jaw. There also may be extremely damaging effects if a person with these implants undergoes an MRI. Nerve damage or tissue infections are some of the possibilities stemming from an MRI.
The Dental Products Panel of the Medical Devices Advisory Committee will meet on July 18 to discuss this topic.
No comments:
Post a Comment